Non-critical medical devices are those in contact with intact skin, but not mucous membranes. Examples of non-critical medical devices vary from stethoscopes, bp cuffs, to Screen & Button electronics, infusion pumps, and wearable monitors and transceivers.¹ Clearly, administrative tools like computer keyboards, mouse, bar-code readers, communicators, and mobile and landline phones fall in the mix of high-touch tools and electronics.
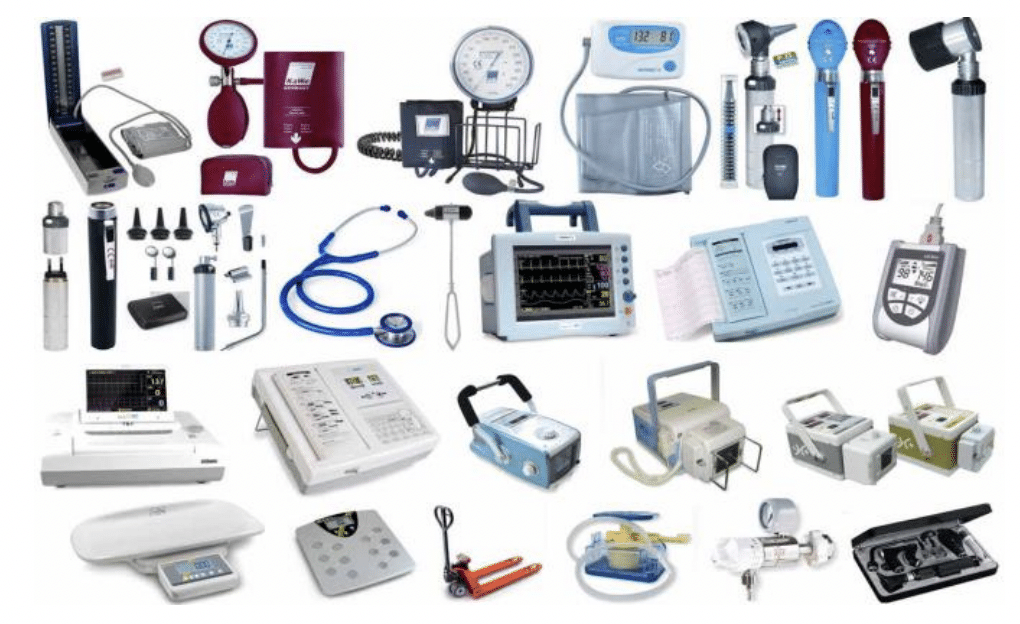
“The literature shows that some of the most-overlooked sources of contamination in healthcare settings are items deemed “non-critical.” ¹’² For example, non-critical items used in patient rooms, such as call buttons, lead wires and oxygen flow meters, are touched throughout the day, but all too often they are not cleaned and disinfected until the patient is discharged.
Thus, such contaminated non-critical items can become a critical source of pathogen transmission, particularly if hand hygiene is poor. When items are labeled “non-critical,” they will be perceived as such—and the importance of cleaning and disinfecting these items will likely take on a low priority.
“For this reason, Livshiz-Riven and coauthors ² have argued that “the concept of ‘non-critical items’ is inappropriate and is an unfortunate term that needs to be changed.” ¹
Item surfaces must first be cleaned for disinfection to be effective. The sole reliance on a disinfectant without physical cleaning is not recommended. When disinfection is required, surfaces or items should always be physically cleaned with a detergent or wipe, followed with a disinfectant.
Cleaning and disinfection of non-critical medical tools is a two-step process. ⁵
1. Cleaning: The physical removal of foreign (e.g., dust, soil) and organic material (e.g., blood, secretions, excretions, microorganisms). Cleaning physically removes rather than kills microorganisms. It is accomplished with water, cleaning agents (e.g., soap/detergents, cleaner/disinfectant wipe) and manual friction.
2. Disinfection: The inactivation of disease-producing microorganisms. Equipment/devices must be cleaned thoroughly before effective disinfection can take place.
a. High-Level Disinfectant (HLD): The level of disinfection required when processing semi-critical medical equipment/devices. High-level disinfection processes destroy vegetative bacteria, mycobacteria, fungi, and enveloped /non-enveloped viruses, but not necessarily bacterial spores.
b. Low-Level Disinfectant (LLD): The level of disinfection required when processing non-invasive medical equipment (e.g., non-critical equipment) and some environmental surfaces.
Operationalize Disinfection of Medical Tools & Electronics
Summary
Non-Critical Medical Tools and Electronics are often overlooked as a source of pathogen transmission.
The large internal capacity, one-touch operation and mobile application of the RDS-32a Rapid UVC Decontamination System, can Operationalize Disinfection with a sanitation protocol that simplifies the disinfection process, eliminates errors, and ensures a certified, HLD result.
The 1-Step RDS Disinfection Protocol, is a guide for disinfecting surface pathogens like VRE, CRE, Candida auris, Candida albicans, MRSA, SARS-CoV2 up to a 6 Log¹⁰ reduction in 30-seconds. ⁶
Patented, Operating Software assures Lab Certified ⁶ and repeatable disinfection results in every cycle by constantly measuring & monitoring the germicidal intensity of the specified UVC Dose.

References:
1] https://pubmed.ncbi.nlm.nih.gov/29281998/
2] https://apic.org/noncritical-is-critical/
3] Livshiz-Riven I, Borer A, Nativ R, Eskira S, Larson E. Relationship between shared patient care items and healthcare-associated infections: a systematic review. Int J Nurs Stud. 2014
4] https://www.health.qld.gov.au/clinical-practice/guidelines-procedures/diseases-infection/infection-prevention/standard-precautions/cleaning-disinfecting
5] https://www.prairiemountainhealth.ca/images/PPG/PPG-00026.pdf
6] Antimicrobial Efficacy, Model RDS-32a on Unopened Packaged Supplies. 30-Seconds to achieve 99.9999 or 6 Log¹° Reduction. Study ID#s: NG 052021, NG 052022, NG 052023
